| S. No. |
Family |
Signature |
Description |
| 1. |
Abaecin |

|
Abaecin represents antimicrobial peptides produced by bees. These peptides have strong antimicrobial and some anti-fungal activity and has homology to abaecin which is the largest proline-rich antimicrobial peptide isolated from European bumblebee Bombus pascuorum [PMID: 9219367]. |
| 2. |
Ascaphin |
|
Ascaphin family of antibacterial peptides are secreted from the frog Ascaphus truei. This family of peptide exhibit no structural similarity with other antimicrobial peptides from frogs skin [PMID: 15207717]. |
| 3. |
Aurein |

|
This family of antibacterial peptides are secreted from the granular dorsal glands of Litoria aurea (Green and golden bell frog), Litoria raniformis (Southern bell frog), Litoria citropa (Australian blue mountains tree frog) and frogs from genus Uperoleia. They are a part of the FSAP peptide family. Amongst the more active of these are aurein 1.2, aurein 2.2 and aurein 3.1; caerin 1.1, maculatin 1.1, uperin 3.6 [PMID: 10951191]; citropin 1.1, citropin 1.2, citropin 1.3 and a minor peptide are wide-spectrum antibacterial peptides [PMID: 10504394]. |
| 4. |
Bacteriocin |
|
Bacteriocins are ribosomally synthesized antimicrobial peptides produced by diverse bacteria. Because of their broad spectrum antimicrobial activity they are widely used both as food preservatives and as next generation therapeutics [PMID: 25186038]. |
| 5. |
Bombinin |

|
Bombinins are animicrobial peptides obtained from the skin secretion of Bombina species. These peptides exhibit broad spectrum antimicrobial activity against Bacteria as well as fungi. [PMID: 19366600] |
| 6. |
Brevinin |
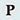
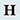
|
Brevinins are antimicrobial peptides obtained from the skin secretions of frogs [PMID: 22307792]. |
| 7. |
Caerin |
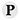

|
This family consists of several caerin 1 proteins from Litoria species, Australian tree frogs. The caerin 1 peptides are among the most powerful of the broad-spectrum antibiotic amphibian peptides [PMID: 12717721]. These peptides are excreted from amphibian skin, and can interact with and disrupt bacterial membranes, leading to the permeabilisation of the cell membrane. |
| 8. |
Cathelicidin |
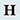
|
The precursor sequences of a number of antimicrobial peptides secreted by neutrophils (polymorphonuclear leukocytes) upon activation have been found to be evolutionarily related and are collectively known as cathelicidins [PMID: 7589491].
Structurally, these proteins consist of three domains: a signal sequence, a conserved region of about 100 residues that contains four cysteines involved in two disulphide bonds, and a highly divergent C-terminal section of variable size. It is in this C-terminal section that the antibacterial peptides are found; they are proteolytically processed from their precursor by enzymes such as elastase. |
| 9. |
Cecropin |

|
Cecropins [PMID: 3318666, PMID: 2015623, PMID: 1915368] are potent antibacterial proteins that constitute a main part of the cell-free immunity of insects. Cecropins are small proteins of about 35 amino acid residues active against both Gram-positive and Gram-negative bacteria. They seem to exert a lytic action on bacterial membranes. Cecropins isolated from insects other than Hyalophora cecropia (Cecropia moth) have been given various names; bactericidin, lepidopteran, sarcotoxin, etc. All of these peptides are structurally related. Cecropin P1, an intestinal antibacterial peptide from Sus scrofa (Pig), also belongs to this family. |
| 10. |
Clavanin |
|
This family consists of clavanin proteins from the haemocytes of the invertebrate Styela clava (Sea squirt), a solitary tunicate. The family is made up of four alpha-helical antimicrobial peptides, clavanins A, B, C and D. The tunicate peptides resemble magainins in size, primary sequence and antibacterial activity. Synthetic clavanin A displays comparable antimicrobial activity to magainins and cecropins. The presence of alpha-helical antimicrobial peptides in the haemocytes of a urochordate suggests that such peptides are primeval effectors of innate immunity in the vertebrate lineage [PMID: 9001389]. |
| 11. |
Coleoptericin |

|
This family consists of several insect coleoptericin, acaloleptin, holotricin and rhinocerosin proteins which are all known to be antibacterial proteins. These all appear to be short, glycine-rich molecules, inducible by infection [PMID: 11520352]. |
| 12. |
Cupiennin |
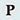
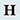
|
Cupiennins are a family of antimicrobial peptides isolated from the venom of Cupiennius salei. The pepides exhibit antimicrobial and insecticidal properties [PMID:11792701]. |
| 13. |
Cyclotide |

|
Cyclotides (cyclo peptides) are plant peptides of ~30 amino acids with a head to-tail cyclic backbone and six cysteine residues involved in three disulphide bonds. The cyclotides are extremely resistant to proteolysis and are remarkably stable. Cyclotides display a diverse range of biological activities, including uterotonic activity, inhibition of neurotensin binding, hemolytic, anti-HIV and anti-microbial activity. This range of biological activities makes cyclotides amenable to potential pharmaceutical and agricultural applications. Although their precise role in plants has not yet been reported, it appears that they are most likely present as defence molecules [PMID: 10600388, PMID: 12482862, PMID: 12482868, PMID: 12946412].
|
| 14. |
Cystatin |
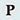
|
Cystatins inhibit cysteine peptidases of the papain family (MEROPS peptidase family C1), and some also inhibit legumain family enzymes (MEROPS peptidase family C13). These peptidases play key roles in physiological processes, such as intracellular protein degradation (cathepsins B, H and L), are pivotal in the remodelling of bone (cathepsin K), and may be important in the control of antigen presentation (cathepsin S, mammalian legumain). Moreover, the activities of such peptidases are increased in pathophysiological conditions, such as cancer metastasis and inflammation. Additionally, such peptidases are essential for several pathogenic parasites and bacteria. Thus in animals cystatins not only have capacity to regulate normal body processes and perhaps cause disease when down-regulated, but in other organisms may also participate in defence against biotic and abiotic stress. |
| 15. |
Defensin |
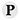

|
Defensins are a large family of antimicrobial peptides. They are small, cationic and exhibit broad spectrum antimicrobial activity by forming pores in the membranes. [PMID: 12949495, 8528769] |
| 16. |
Dermaseptin |
|
Dermaseptin proteins are used in eukaryotes as an antimicrobial agent. Dermaseptins are typically between 30 to 76 amino acids in length. This domain is found associated with PF03032. The full-length protein is almost completely defined in an alpha helical domain. It creates high levels of disorder at the level of the phospholipid head group of bacterial membranes suggesting that it partitions into the bilayer where it severely disrupts membrane packing.
The polypeptide precursor to the dermaseptins consist of a tripartite organisation: a N-terminal signal peptide, followed by an acidic region terminated by two basic Lys-Arg residues and the actual dermaseptin at the C terminus. |
| 17. |
Esculentin |

|
Esculentins are antimicrobial peptides obtianed from the skin secretions of frogs [PMID: 22307792]. |
| 18. |
Formaecin |
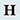
|
Formaecin family of antimicrobial peptides isolated from the bulldog ant Myrmecia gulosa in response to bacterial infection. Formaecins are inducible peptide antibiotics and are active against growing Escherichia coli but were inactive against other Gram-negative and Gram-positive bacteria. Formaecin peptides are 16 amino acids long, are rich in proline and have N-acetylgalactosamine O-linked to a conserved threonine [PMID: 9497332]. |
| 19. |
Gaegurin |

|
Gaegurins exibit strong antimicrobial activity against Gram positive and negative bacteria, fungi and protozoa. These peptides have little or no hemolytic activity which makes them potential therapeutic agents [PMID: 7999137] |
| 20. |
Halocidin |
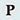
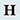
|
Halocidins are isolated from the haemocytes of the tunicate, Halocynthia aurantium (Sea peach). They are dimeric in structures, which are found via a disulphide linkage between cysteines of two different- sized monomers. Halocidins have been shown to have strong antimicrobial activities against a wide variety of pathogenic bacteria and could be ideal candidates as peptide antibiotics against multidrug-resistant bacteria [PMID: 12067731]. |
| 21. |
Hepcidin |

|
Hepcidin is a antibacterial and anti-fungal protein expressed in the liver and is also a signalling molecule in iron metabolism. The hepcidin protein is cysteine-rich and forms a distorted beta-sheet with an unusual disulphide bond found at the turn of the hairpin [PMID: 12138110]. |
| 22. |
Histatin |
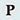
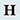
|
This family consists of antimicrobial peptides that are rich in histidines. These are peptides found in the saliva and exert antifungal activities [PMID: 18650243]. |
| 23. |
Latarcin |

|
This entry represents the precursor proteins for a number of short antimicrobial peptides called Latarcins. Latarcins were discovered in the venom of the spider Lachesana tarabaevi [PMID: 16735513]. Latarcins are likely to adopt amphipathic alpha-helical structure in the plasma membrane. |
| 24. |
LEAP-2 |
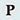
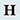
|
This family consists of several mammalian liver-expressed antimicrobial peptide 2 (LEAP-2) sequences. LEAP-2 is a cationic cysteine-rich protein. LEAP-2 contains a core structure with two disulfide bonds formed by cysteine residues in relative 1-3 and 2-4 positions. LEAP-2 is synthesised as a 77-residue precursor, which is predominantly expressed in the liver and highly conserved among mammals. The largest native LEAP-2 form of 40 amino acid residues is generated from the precursor at a putative cleavage site for a furin-like endoprotease. In contrast to smaller LEAP-2 variants, this peptide exhibits dose-dependent antimicrobial activity against selected microbial model organisms [PMID: 12493837]. |
| 25. |
Maximin |
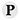

|
This family consists of peptides from Bombina maxima (Giant fire-bellied toad). Two groups of antimicrobial peptides have been isolated from skin secretions of B. maxima. Peptides in the first group, named maximins 1, 2, 3, 4 and 5, are structurally related to bombinin-like peptides (BLPs). Unlike BLPs, sequence variations in maximins occurred all through the molecules. In addition to the potent antimicrobial activity, cytotoxicity against tumour cells and spermicidal action of maximins, maximin 3 possessed a significant anti-Simian-Human immunodeficiency virus (HIV) activity. Maximins 1 and 3 have been found to be toxic to mice. Peptides in the second group, termed maximins H1, H2, H3 and H4, are homologous with bombinin H peptides [PMID: 11835991]. |
| 26. |
Melittin |
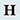
|
Melittin is the principal protein component of the venom of the honeybee, Apis mellifera. It inhibits protein kinase C, Ca2+/calmodulin-dependent protein kinase II, myosin light chain kinase and Na+/K+-ATPase (synaptosomal membrane) and is a cell membrane lytic factor. Melittin is a small peptide with no disulphide bridge; the N-terminal part of the molecule is predominantly hydrophobic and the C-terminal part is hydrophilic and strongly basic.
The molecular mechanisms underlying the various effects of melittin on membranes have not been completely defined and much of the evidence indicates that different molecular mechanisms may underlie different actions of the peptide [PMID: 2187536].
Extensive work with melittin has shown that the venom has multiple effects, probably, as a result of its interaction with negatively changed phospholipids. It inhibits well known transport pumps such as the Na+-K+-ATPase and the H+-K+-ATPase. Melittin increases the permeability of cell membranes to ions, particularly Na+ and indirectly Ca2+, because of the Na+-Ca2+-exchange. This effect results in marked morphological and functional changes, particularly in excitable tissues such as cardiac myocytes. In some other tissues, e.g., cornea, not only Na+ but Cl- permeability is also increased by melittin. [PMID: 10072885].
The study of melittin in model membranes has been useful for the development of methodology for determination of membrane protein structures. A molecular dynamics simulation of melittin in a hydrated dipalmitoylphosphatidylcholine (DPPC) bilayer was carried out. The effect of melittin on the surrounding membrane was localised to its immediate vicinity, and its asymmetry with respect to the two layers may be a result of the fact that it is not fully transmembranal. Melittin's hydrophilic C terminus anchors it at the extracellular interface, leaving the N terminus "loose" in the lower layer of the membrane [PMID: 10692322]. |
| 27. |
Metchnikowin |
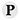

|
This family consists of the metchnikowin family of antimicrobial peptides from Drosophila. Metchnikowin is a proline-rich peptide whose expression is immune-inducible. Induction of the metchnikowin gene expression can be mediated either by the TOLL pathway or by the imd gene product. The metchnikowin peptide is unique among the Drosophila antimicrobial peptides in that it is active against both bacteria and fungi [PMID: 9600835]. |
| 28. |
Moricin |
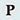
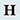
|
Moricin is a antibacterial peptide that is highly basic. The structure of moricin reveals that it is comprised of a long alpha-helix. The N terminus of the helix is amphipathic, and the C terminus of the helix is predominately hydrophobic. The amphipathic N-terminal segment of the alpha- helix is mainly responsible for the increase in permeability of the bacterial membrane which kills the bacteria [PMID: 11997013]. |
| 29. |
Myticin |

|
Myticin is a cysteine-rich peptide produced in three isoforms, A, B and C, by Mytilus galloprovincialis, the Mediterranean mussel. Some isoforms show antibacterial activity against gram-positive bacteria, while others are additionally active against the fungus Fusarium oxysporum and a gram-negative bacterium, Escherichia coli D31. Myticin-prepro is the precursor peptide. The mature molecule, named myticin, consists of 40 residues, with four intramolecular disulfide bridges and a cysteine array in the primary structure different from that of previously characterised cysteine-rich antimicrobial peptides. The first 20 amino acids are a putative signal peptide, and the antimicrobial peptide sequence is a 36-residue C-terminal extension. Such a structure suggests that myticins are synthesised as prepro-proteins that are then processed by various proteolytic events before storage in the haemocytes as the active peptide. Myticin precursors are expressed mainly in the haemocytes. The family Mytilin has been merged into this family [PMID: 10491159] |
| 30. |
Ocellatin |
|
This family consists of the ocellatin family of antimicrobial peptides. Ocellatins are produced from the electrical-stimulated skin secretions of the South American frog, Leptodactylus ocellatus (Argus frog). The family consists of three structurally related peptides, ocellatin 1, ocellatin 2 and ocellatin 3. These peptides present haemolytic activity against human erythrocytes and are also active against Escherichia coli [PMID: 15648972]. |
| 31. |
Palustrin |

|
Palustrins are antimicrobial peptides obtianed from the skin secretions of frogs [PMID: 21911019, 11087945]. |
| 32. |
Penaeidin |
|
Penaeidins are unique family of antimicrobial peptides which were initially identified in the hemolymph of the Pacific white shrimp, Penaeus vannamei. These peptides contain an N-terminal termianl proline-rich and a C- terminal cysteine-rich domains [PMID: 12242595]. |
| 33. |
Phylloseptin |

|
This family consists of antimicrobial peptides that are a group of structurally related peptides identified in the skin secretions of the Brazilian tree frog Phyllomedusa hypochondrialis and P. oreades [PMID: 15752569]. |
| 34. |
Plantazolicin |
|
Members of this protein family are precursors of TOMMs, that is, thizazole/oxazole-modified microcins. Members are about 42 residues in length, have a C-terminal region of extremely low complexity rich in Ser, and are often missed by ab initio gene callers. In the dimethylated form plantazolicin A, Bacillus amyloliquefaciens plantazolicin has the antibiotic activity of inhibiting growth of Gram-positive bacteria. The desmethyl form, plantazolicin B, has no antibiotic activity. The mode of action appears to be disruption of cell walls and lysis of cells. [PMID: 20971906, PMID: 21950656]. |
| 35. |
Pleurocidin |

|
This family consists of the pleurocidin family of antimicrobial peptides. Pleurocidins are found in the skin mucous secretions of the winter flounder (Pleuronectes americanus) and these peptides exhibit antimicrobial activity against Escherichia coli. Pleurocidin is predicted to assume an amphipathic alpha-helical conformation similar to other linear antimicrobial peptides and may play a role in innate host defence [PMID: 9115266]. |
| 36. |
Ponericin |
|
This family contains a number of ponericin peptides (approximately 30 residues long) from the venom of the predatory ant Pachycondyla goeldii (Ponerine ant). These peptides exhibit antibacterial and insecticidal properties, and may adopt an amphipathic alpha-helical structure in polar environments such as cell membranes [PMID: 11279030]. |
| 37. |
Pseudin |

|
Pseudins are a subfamily of the FSAP family (Frog Secreted Active Peptides) extracted from the skin of the paradoxical frog Pseudis paradoxa. The pseudins belong to the class of cationic, amphipathic-helical antimicrobial peptides [PMID: 11689009]. |
| 38. |
Ranatuerin |
|
Ranatuerins are antimicrobial peptides obtained from skin secretions of frog [PMID: 9784389]. |
| 39. |
Rugosin |

|
Rugosins are antimicrobial peptides obtained from the skin secretions of frogs [PMID: 7612013]. |
| 40. |
Tachystatin |
|
Tachystatins are antimicrobial peptides identified from the horseshoe crab Tachypleus tridentatus. This family of antimicrobial peptides exhibit broad spectrum acivity against bacteria as well as fungi [PMID: 10473569]. |
| 41. |
Temporin |

|
Temporins show some sequence similarity to hemolytic peptides isolated from Vespa venom [PMID: 6206053]. Natural and synthetic temporins have antibacterial activity against gram-positive bacteria with no hemolytic activtiy. Temporins are one of the smallest antibacterial peptides found in nature [PMID: 9022710]. |
| 42. |
Thaumatin |
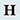
|
Thaumatin consists of about 200 residues and contains 8 disulphide bonds. Like other PR proteins, thaumatin is predicted to have a mainly beta structure, with a high content of beta-turns and little helix [PMID: 7049841]. Several stress-induced proteins of plants have been found to be related to thaumatins:A maize alpha-amylase/trypsin inhibitor, two tobacco pathogenesis-related proteins: PR-R major and minor forms,which are induced after infection with viruses, Salt-induced protein NP24 from tomato, Osmotin, a salt-induced protein from tobacco [PMID: 16666857], Osmotin-like proteins OSML13, OSML15 and OSML81 from potato [PMID: 7630973], P21, a leaf protein from soybean, PWIR2, a leaf protein from wheat [PMID: 1650615], Zeamatin, a maize antifunal protein [PMID: 7846159]. This protein is also referred to as pathogenesis-related group 5 (PR5), as many thaumatin-like proteins accumulate in plants in response to infection by a pathogen and possess antifungal activity [PMID: 1463856]. The proteins are involved in systematically acquired resistance and stress response in plants, although their precise role is unknown [PMID: 1463856]. |
| 43. |
Thionin |
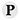

|
Thionins are small, basic plant proteins, 45 to 50 amino acids in length, which include three or four conserved disulphide linkages. The proteins are toxic to animal cells, presumably attacking the cell membrane and rendering it permeable: this results in the inhibition of sugar uptake and allows potassium and phosphate ions, proteins, and nucleotides to leak from cells [PMID: 3985614]. Thionins are mainly found in seeds where they may act as a defence against consumption by animals. A barley (Hordeum vulgare) leaf thionin that is highly toxic to plant pathogens and is involved in the mechanism of plant defence against microbial infections has also been identified [PMID: 1377959]. The hydrophobic protein crambin from the Abyssinian kale (Crambe abyssinica) is also a member of the thionin family [PMID: 3985614]. |
| 44. |
Transferrin |
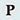
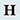
|
Transferrins are eukaryotic iron-binding glycoproteins with the ability to bind ferric iron with high affinity in the cleft of each of two homologous lobes. They control the level of free iron in biological fluids [PMID: 3032619]. Members of the family include blood serotransferrin (siderophilin), milk lactotransferrin (lactoferrin), egg white ovotransferrin (conalbumin) and membrane-associated melanotransferrin. The iron-deficient transferrins inhibit the growth of certain bacteria and fungi by making iron unavailable for bacterial metabolism [PMID: 6452038]. |
| 45. |
Uperin |

|
This family consists of wide-spectrum antibiotic peptides isolated from the Australian toadlet, Uperoleia mjobergii. Uperins are smaller than most other wide-spectrum antibiotic peptides isolated from amphibians. Uperins adopts a well-defined amphipathic alpha-helix with distinct hydrophilic and hydrophobic faces [PMID: 10461748]. |
